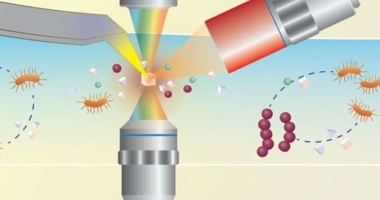
Researchers have developed a sensitive technique that can analyze viral nucleic acids in just 20 minutes using “glow-in-the-dark” proteins. Bioluminescence, powered by the luciferase protein, has been integrated into sensors to detect their target, but the sensors have not achieved the exceptional sensitivity necessary for clinical diagnostic tests. However, Maarten Merkx and colleagues have combined CRISPR-related proteins with bioluminescence to make use of CRISPR’s gene-editing technique, with a digital camera detecting the signal. The researchers performed recombinase polymerase amplification (RPA), a simple method that works at a constant temperature of about 100 F, to ensure that there was enough sample RNA or DNA to analyze. The new technique, called LUNAS (luminescent nucleic acid sensor), uses two CRISPR/Cas9 proteins specific for different neighboring parts of a viral genome, each with a distinct fragment of luciferase attached to them. RPA-LUNAS successfully detected SARS-CoV-2 RNA within 20 minutes, even at concentrations as low as 200 copies per microliter when tested on clinical samples collected from nasal swabs. The LUNAS assay has great potential for detecting many other viruses effectively and easily. The study was funded by the Dutch Research Council | Nationaal Regieorgaan Praktijkgericht Onderzoek SIA (NRPO-SIA) and the Eindhoven University Fund.
Diagnostic tests for viral diseases have seen significant advancements, but many highly sensitive tests still rely on complex sample preparation and result interpretation methods. This renders them unsuitable for resource-limited areas or point-of-care settings. In ACS Central Science, researchers reveal a sensitive technique that can analyze viral nucleic acids in just 20 minutes using a one-step process with “glow-in-the-dark” proteins.
Bioluminescence is powered by a chemical reaction involving the luciferase protein, the scientific phenomenon behind the firefly’s glow, the anglerfish’s radiant lure, and the ghostly blue of phytoplankton-laden shores. This luminescent protein has been integrated into sensors that emit visible light when detecting their target. However, until now, these sensors have not achieved the exceptional sensitivity necessary for clinical diagnostic tests.
Maarten Merkx and colleagues combined CRISPR-related proteins with a bioluminescence technique to make use of CRISPR’s gene-editing technique, but with just a digital camera to detect its signal. The researchers performed recombinase polymerase amplification (RPA), a simple method that works at a constant temperature of about 100 F to ensure that there was enough sample RNA or DNA to analyze. With the new technique, called LUNAS (luminescent nucleic acid sensor), two CRISPR/Cas9 proteins specific for different neighboring parts of a viral genome each have a distinct fragment of luciferase attached to them.
If a specific viral genome that the researchers were testing for was present, the two CRISPR/Cas9 proteins would bind to the targeted nucleic acid sequences, allowing the complete luciferase protein to form and shine blue light in the presence of a chemical substrate. A tube that changed from green to blue indicated a positive result.
RPA-LUNAS successfully detected SARS-CoV-2 RNA within 20 minutes, even at concentrations as low as 200 copies per microliter, when tested on clinical samples collected from nasal swabs. The researchers say that the LUNAS assay has great potential for detecting many other viruses effectively and easily.
Funding for “Glow-in-the-Dark” Infectious Disease Diagnostic Research
The research on “glow-in-the-dark” infectious disease diagnostics using CRISPR-Cas9-based split luciferase complementation was funded by the Dutch Research Council | Nationaal Regieorgaan Praktijkgericht Onderzoek SIA (NRPO-SIA) and the Eindhoven University Fund.
Don’t miss interesting posts on Famousbio