Scientists from the University of Chicago have discovered a new and easier method for creating MXenes with fewer toxic byproducts. This method involves mixing several chemicals with the desired metal and heating the mixture at 1,700°F. MXenes are a new material with promise for new applications in high-tech electronics or energy storage, consisting of many extremely thin layers of metal. They can retain the special abilities of metal, such as conducting electricity strongly, while being easily customizable. These advantages make MXenes potentially very useful for building new devices, such as those used for storing energy or blocking electromagnetic wave interference. The team tested the method with titanium and zirconium metals but believes it can also be used for many other different combinations.
Scientists Discover New Method to Create Atomically-Thin Metal Layers with Fewer Byproducts
MXenes, a new material with promise for new applications in high-tech electronics or energy storage, consists of many extremely thin layers of metal. These layers can be used to slip different ions for various purposes, making them potentially very useful for future innovation. However, until recently, these materials were as labor-intensive as good croissants made in a French bakery.
A breakthrough by scientists with the University of Chicago shows a new, more efficient and less toxic way to create MXenes. The discovery, published in Science, involves the principles of chemistry, particularly “atom economy,” which seeks to minimize the number of wasted atoms during a reaction.
When MXenes were discovered in 2011, scientists were excited because shaving a metal like gold or titanium to create atomic-thin sheets usually causes it to stop behaving like a metal. But the unusually strong chemical bonds in MXenes allow them to retain the special abilities of metal, like conducting electricity strongly.
MXenes are also easily customizable, allowing ions to be put between the layers for various purposes. Chemistry graduate student Di Wang and postdoctoral scholar Chenkun Zhou, co-first authors of the paper, said MXenes could be used to store energy or block electromagnetic wave interference.
The team’s previous method of creating MXenes involved several intensive chemical engineering steps, including heating the mixture at 3,000°F followed by a bath in hydrofluoric acid. Although it is fine for making a few grams for laboratory experiments, it is a major corrosive waste disposal issue if you wanted to make large amounts for commercial products.
The new method discovered by the team is simple and inexpensive. It consists of just one step: mixing several chemicals with the desired metal, then heating the mixture at 1,700°F. This method results in fewer toxic byproducts than the previous method.
The discovery of this new method to create MXenes may pave the way towards using them in everyday electronics and devices. Talapin, the corresponding author on the paper, said it could lead to new innovation and development of commercial products.
Easier and Less Toxic Method for Creating MXenes
Scientists from the University of Chicago have discovered a new method for creating MXenes, which involves fewer toxic byproducts and is more efficient. The method involves mixing several chemicals with the desired metal and heating the mixture at 1,700°F, resulting in MXenes with many extremely thin layers of metal.
This new method paves the way for the exploration and creation of new varieties of MXenes for different applications. The team tested the method with titanium and zirconium metals but believes it can also be used for many other different combinations.
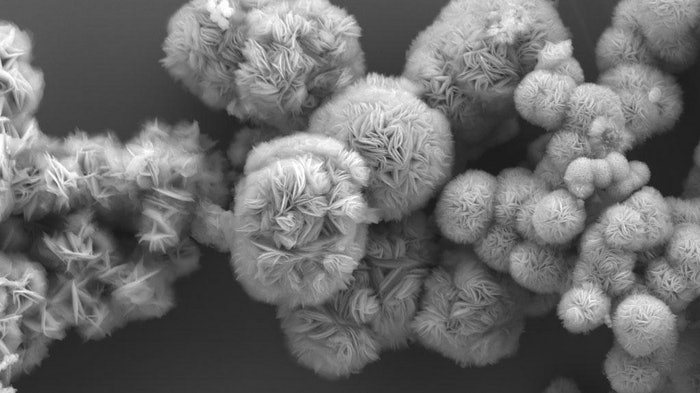
MXenes created using this method also have a visually appealing structure. They stand up like flowers, which makes them better for reactions because the edges are exposed and accessible for ions and molecules to move in between the metal layers.
The research was conducted with the help of UChicago colleagues across departments and facilities, including theoretical chemist Suri Vaikuntanathan, X-ray research facility director Alexander Filatov, and electrochemists Chong Liu and Mingzhan Wang of the Pritzker School of Molecular Engineering. Electron microscopy was performed by Robert Klie and Francisco Lagunas with the University of Illinois Chicago.
This research was partly conducted via the U.S. Department of Energy’s Advanced Materials for Energy-Water Systems, an Energy Frontier Research Center, the University of Chicago Materials Research Science and Engineering Center, and the Center for Nanoscale Materials at Argonne National Laboratory.
Don’t miss interesting posts on Famousbio