Fungi-generated enzyme Laccase has shown substrate promiscuity in degrading various hazardous organic dye molecules, particularly those drained into water bodies from textile industries. Scientists discovered that this enzyme can degrade many organic dye molecules with varying kinetics, charge, size, and shape, using redox reactions to produce only water and less virulent oxides of carbon, nitrogen, and sulfur. This discovery can lead to the development of an eco-friendly solution for treating heavily dye-polluted water using enzyme-coated cassettes. The active site of laccase can accommodate a wide range of dye molecules due to the conformational plasticity of a loop covering the active site, making it an excellent broad-spectrum degrader for industrial dye effluents.
Fungi-generated enzyme Laccase has been found capable of breaking down hazardous organic dye molecules. These dyes are commonly disposed of in water bodies after being used in textile industries. The characteristic that allows this enzyme to degrade various organic molecules is called substrate promiscuity. Scientists see a potential in developing an enzyme-coated cassette for treating heavily dye-polluted water, offering a natural solution to reduce environmental pollution.
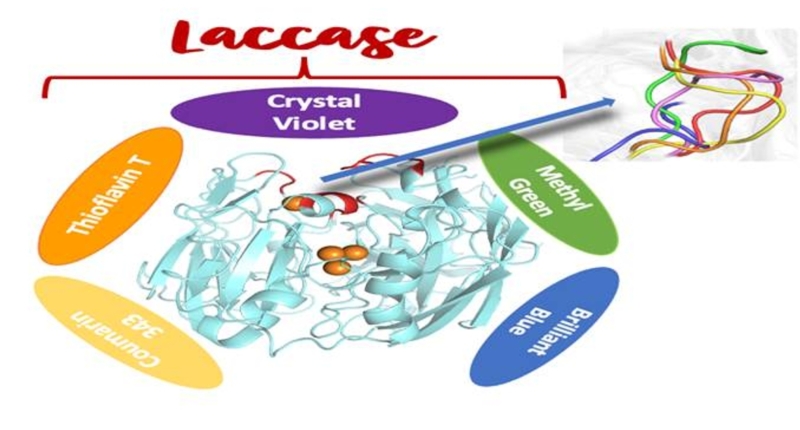
Prof. Ranjit Biswas and Dr. Suman Chakrabarty from the S. N. Bose National Center for Basic Sciences (SNBNCBS), Kolkata, tested the efficacy of laccase in degrading some standard dye molecules such as Methyl Green, Crystal Violet, Thioflavin T, Coumarin 343, and Brilliant Blue. They found that laccase, generated by a group of fungi, can degrade many organic dye molecules with varying kinetics, charge, size, and shape, using redox reactions to produce only water and less virulent oxides of carbon, nitrogen, and sulfur.
The scientists have elucidated the molecular thermodynamic origin and mechanism behind this substrate promiscuity using computational modeling and simulation. They found that the active site of laccase can accommodate a wide range of dye molecules with varying charge and shape due to the conformational plasticity of a loop covering the active site. The shape of the binding pocket can change adaptively. Internal cancellation between different types of interactions leads to almost similar binding affinity for very different molecules. This substrate promiscuity of laccase offers an immense biotechnological potential for a broad-spectrum degrader for industrial dye effluents.
The scientists combined UV/Visible spectroscopy and computer simulations to demonstrate the versatility of laccase in breaking down organic dye molecules. This characteristic can have deep implications in designing enzyme-coated cassettes for treating heavily dye-polluted water, thereby providing a natural solution to make the environment greener.
To conclude, laccase has shown substrate promiscuity in degrading various hazardous organic dye molecules. The ability of the enzyme to break down these molecules through redox reactions offers an eco-friendly solution to reducing environmental pollution caused by the textile industry. This discovery can have significant implications in developing a natural solution to treat heavily dye-polluted water.
Don’t miss interesting posts on Famousbio